What is a Water Softener?
A water softener is an appliance that is fitted to the mains water supply to a home or commercial premises to remove dissolved minerals (hardness salts). These minerals form lime-scale in pipes and boilers, when the water is heated, and soap-scum in bath water, laundry appliances and sanitary-ware. They are usually fitted close to the mains water entry point in a house but can be sited almost anywhere. Modern domestic softeners are so compact that they can be easily installed under the kitchen sink.
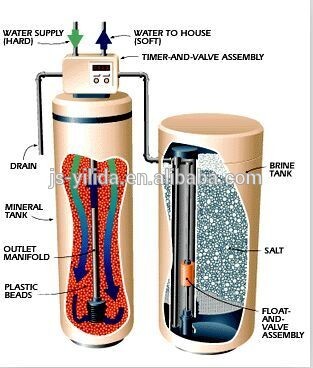
What does a Water Softener do?
Water softeners use a process called ion exchange. The softener unit contains a column filled with beads of a resin which remove the dissolved calcium and magnesium ions from the hard water flowing through it, replacing them with sodium. Periodically, (typically once a day) the softener regenerates the resin by washing with brine (sodium chloride solution), carrying the calcium and magnesium to drain, and leaving fresh sodium ions on the resin ready for the next treatment cycle. Regeneration is usually triggered automatically by either an electronic timer or mechanical water flow meter. The unit has to be refilled with salt (in the form of granules, small tablets or blocks) typically once a month.
Water suppliers define the level of water hardness (total hardness) in mg/l (milligrams per litre) of calcium carbonate. The water supply is considered to be hard if it is above 200 mg/l. 60% of water supplies in the UK are hard and are typically 300 to 350 mg/l total hardness. Softening generally reduces the hardness to less than 10 mg/l.

Softeners are supplied in different shapes and sizes. All have a tank to hold the resin through which the water is passed to soften it, all have a tank to store and dissolve the salt for the regenerating brine and all softeners regenerate automatically. There are various ways in which softeners determine when to regenerate - some are fitted with a timer and some regenerate after a pre-set volume of water has been treated.
Ion exchange involves the use of a resin bed. Ion exchange resin is a very small synthetic bead-like material that looks a little bit like brown sugar. The beads are very small, about the size of a pin head.
The process is called Ion exchange because the ions calcium (Ca) and magnesium (Mg) are exchanged for small amounts of sodium (Na).
Water softeners use ion exchange (see above) and although they all differ in design they do share the following characteristics.
working principle of sodium ion exchange and softening treatment is to exchange sodium cation with resin to soften the raw water , hardness content Ca2+and Mg2+ in water will be exchanged with Na+ in the resin to absorb the Ca2+and Mg2+ to make the water to be softened.
2RNa + Ca2+ = R2Ca + 2Na+
2RNa + Mg2+ = R2Mg + 2Na+
After the water treated by sodium ion exchanger, the Ca+ and Mg+ were exchanged to
be Na +.
Application
It is widely used in industrial water and civil water softening treatment, such as water supply for boiler feeding water and air conditioning system water, heat exchanger, power plant, chemical, textile, printing and dyeing, bio-pharmaceutical, Electronic system and water pretreatment.
One work one standby type: one tank is running while the other is alternating, when water yield of operating tank reaches the presetted flow rate, it will start the regeneration , meanwhile, the other tank will start working, these two tanks work and regenerate alternatively to realize continuous 24 hours water supplying.








 China
China



